Complete Story
Coronavirus (COVID-19) Resources
Pharmacists are the most accessible healthcare providers in the U.S. and are on the front lines of the current coronavirus disease 2019-2020 outbreak. The Ohio Pharmacists Association is working with state leaders and closely monitoring developments related to COVID-19 from both the Centers for Disease Control and Ohio Department of Health. We will update this resource page as new resources and news becomes available.
Use this one page summary document created by Harvard Medical School students to review the virology and basic background of COVID-19. As patients are asking questions regarding COVID-19 symptoms, use this guide to help you differentiate between COVID-19, seasonal flu, a cold and seasonal allergies.
Help OPA Promote the Importance of Pharmacist Care During this Pandemic
As pharmacists are on the front lines working to help contain the pandemic, providing patient care, and ease the concerns and fears of the general public. Ohio Pharmacists Association created a short survey to help tell your story. Please fill out this survey to help OPA share the amazing work you are doing to the general public, other organizations and social media.
COVID-19 Vaccine Provider Sign Up
Interested providers are requested to voluntarily sign up to be recognized as a COVID-19 vaccine provider. The state of Ohio will not require that all providers offer the vaccine once it is available to the public. Additionally, the state is not requiring that Ohio residents get vaccinated with the COVID-19 vaccine once available. A timeline of the vaccine release date was shared with a predicted release in mid-November for a two-dose COVID-19 vaccine series. The vaccines will be delivered in kits with a minimum of 100 doses per kit.
OPA COVID-19 Pharmacy Relief Registry
OPA has been contacted by members about their shortage of pharmacy team members during the COVID-19 outbreak. To assist pharmacy staffing needs, we are working to develop the OPA COVID-19 Pharmacy Relief Registry.
If you are a pharmacist, pharmacy intern, or registered/certified pharmacy technician who is willing to temporarily assist another practice site in Ohio, please register using the button below. By providing your information, you agree to have your name, city, email, and phone number listed on the Members Only page of the OPA website to be contacted to fill staffing needs.
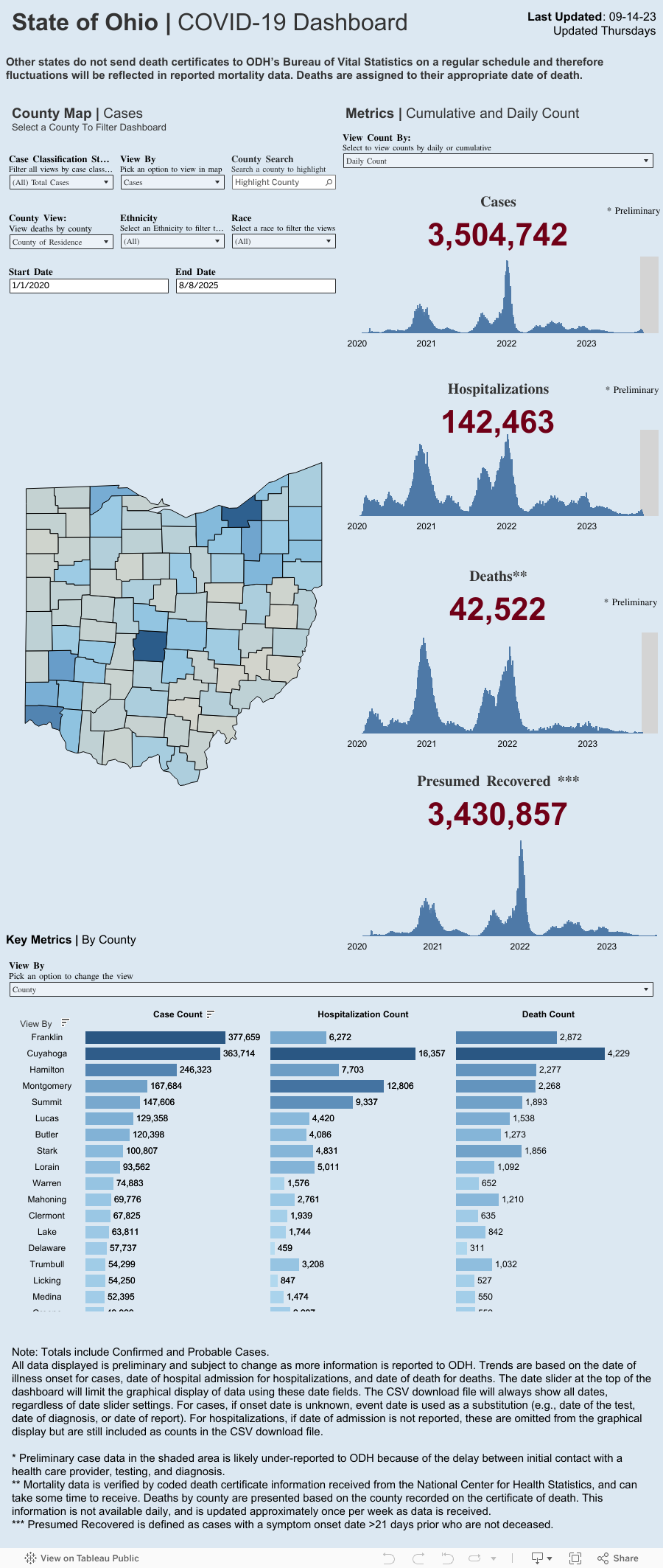
This interactive map is adopted from “Johns Hopkins Center for Systems Science and Engineering”.
Ohio Department of Health
The Ohio Department of Health will answer questions from the public regarding coronavirus (COVID-19) at 1-833-4-ASK-ODH (1-833-427-5634). For the most up-to-date information about COVID-19 and resources visit coronavirus.ohio.gov.
The Ohio Department of Health has many resources and guidance documents to help healthcare providers care for the ill and mitigate their own risk. Read the guidance documents here.
- Updated COVID-19 Testing Guidelines
- COVID Testing Sign Up
- Enrollment Checklist to review before signing up
- COVID-19 Vaccine Enrollment
- COVID-19 Vaccination Program Provider Agreement Frequently Asked Questions (FAQs)
- COVID-19 Vaccine Program Provider Enrollment Webinar
- COVID-19 Vaccine Program Provider Enrollment PowerPoint
- Website to enroll as a COVID-19 vaccine provider: https://ohid.ohio.gov/wps/portal/gov/ohid/
- Local Health District Information
- Resources for Healthcare Providers
Public Health and Executive orders signed by the Governor and Health Director in response to COVID-19
Governor DeWine Executive Orders
- Updated orders can be found here.
Ohio Department of Health
- Order to Prohibit Mass Gatherings in the State of Ohio, with Exceptions
- Updated public health orders can be found here.
State of Ohio Board of Pharmacy
- Vaccine Storage and Administration in Institutional Facilities and Other Settings
- Vaccine Administration and COVID-19 Testing
Insurance
Ohio Department of Insurance
If you have questions regarding Medicare beneficiaries please visit Medicare.gov or call 800-Medicare (800-633-4227). For Medicare caregiver questions or concerns, please visit the Ohio Department of Aging website or call 866-243-5678 to find the Area Agency on Aging serving your community.
- Updated information can be found here.
Pharmacy Benefit Manager Changes
For the most up-to-date information about COVID-19 and the changes from pharmacy benefit managers visit the following websites:
- CVS Caremark
- Express Scripts
- HMSA, an independent licensee of the Blue Cross and Blue Shield Association
- Independent Health
- OptumRx
- Prime Therapeutics Frequently Asked Questions Document
Resources
National Resources
- Centers for Disease Control and Prevention (CDC)
- Coronavirus Main Page
- Information for Healthcare Professionals about Coronavirus (COVID-19)
- CDC Guidance for Pharmacists during COVID-19
- Strategies for Optimizing the Supply of PPE
- Recommendations for Health Care Professionals with Potential Exposure in a Healthcare Setting
- CDC Guidance on People at High Risk for COVID-19
- Severe Illness Associated with Using Non-Pharmaceutical Chloroquine Phosphate to Prevent and Treat Coronavirus Disease 2019 (COVID-19)
- CDC Guidance for Pharmacists during COVID-19
- CDC Guidance for Healthcare Personnel Returning to Work after Confirmed or Suspected COVID-19
- Weekly Summary
- Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19)
- Centers for Medicare & Medicaid Services (CMS)
- Coronavirus Main Page
- Guidance for All Medicare Advantage Organizations, Part D Sponsors, and Medicare-Medicaid Plans
- Flexibilities for telehealth
- Health Care Providers Fact Sheet
- CMS Coronavirus Toolkit
- Regulations For Pharmacist COVID-19 Testing
- Medicare Pharmacies and Other Suppliers May Temporarily Enroll as Independent Clinical Diagnostic Laboratories to Help Address COVID-19 Testing
- How to Obtain a CLIA Certificate of Waiver
- Drug Enforcement Administration (DEA)
- Coronavirus Main Page
- Guidance on Oral CII Prescriptions
- Guidance on Prescribing
- Guidance on Early Refills for Controlled Medications
- Exception to Separate Registration Requirements Across State Lines
- Guidance on Permitting Use of Unregistered Off-Site Locations to Provide Off-Site MAT Services
- Guidance on Satellite Hospital/Clinic Registration Exception
- Guidance on 5% Rule Exception
- Use of Telephone Evaluations to Initiate Buprenorphine Prescribing
- Federal Communications Commission
- Food and Drug Administration (FDA)
- Coronavirus Main Page
- List of All Current COVID-19 Authorized Tests
- Guidance of REMS Program
- OPA has created a one-page summary document outlining the FDA update on clozapine dispensing in response to COVID-19.
- Temporary Policy for Compounding of Certain Drugs for Hospitalized Patients by Outsourcing Facilities During the COVID-19 Public Health Emergency
- COVID-19 Social Media Toolkit
- Temporary Policy Regarding Non-Standard PPE Practices for Sterile Compounding by Pharmacy Compounders not Registered as Outsourcing Facilities During the COVID-19 Public Health Emergency
- FAQs on Testing for SARS-CoV-2
- Coronavirus Disease 2019 (COVID-19) Resources for Health Professionals
- FDA COVID-19 Response
- Coronavirus Treatment Acceleration Program
- Health Resources & Services Administration (HRSA)
- Joint Commission
- Coronavirus Main Page
- Joint Commission Statement on Removing Barriers to Mental Health Care
- Preventing Coronavirus Transmission in Ambulatory Health Care Settings
- Resilience for Second Victims During and After COVID 19
- Quick Safety Issue 54: Promoting psychosocial well-being of health care staff during crisis
- COVID-19 Health Care Staff Trauma and Resilience Oriented Healing
- Occupational Safety and Health Administration (OSHA)
- U.S. Department of Health and Human Services (HHS)
- Coronavirus Main Page
- Trump Administration Partners with Chain and Independent Community Pharmacies to Increase Access to Future COVID-19 Vaccines
- HHS Expands Access to Childhood Vaccines during COVID-19 Pandemic
- Access to Vaccines, COVID-19 Tests
- Guidance Authorizing Pharmacists to Order and Administer COVID-19 Tests
- Telehealth
- Community-Based Testing Sites for COVID-19
- HHS: Advisory Opinion Clarifying Pharmacists’ COVID-19 Testing in All States
National Pharmacy Resources
- American Pharmacists Association (APhA)
- American Society of Health-System Pharmacists (ASHP)
- National Community Pharmacists Association (NCPA)
Ohio Resources
- Ohio Department of Mental Health and Addiction Services
- Coronavirus Main Page
- Ohio Launches Toll-Free ‘COVID Careline’ to Provide Emotional Support for Ohioans: 1-800-720-9616
- Ohio Department of Medicaid
- Ohio Poison Control
- Ohio State Medical Association
General COVID-19 Resources
- World Health Organization (WHO)
- Battelle Decontamination System Information
- National Association of Specialty Pharmacy is providing comprehensive COVID-19 resources
Patient Resources
- CDC Fact Sheet
- CDC What to do if you are sick with COVID-19
- CDC Manage Your COVID-19 Symptoms at Home
- CDC/NCPA Stop the Spread of Germs
- CDC Information for Travel
- CDC Frequently Asked Questions
- The President's Coronovirus Guidelines for the U.S.
- Talking to Your Kids about COVID-19
- Helping Children Cope with Changes Resulting from COVID-19
- FDA Patient Resources
- COVID-19 | Five Things About Staying Mentally Healthy During the COVID-19 Outbreak
- COVID-19 One-Stop Shop Toolkits
- Celebrating Thanksgiving


